QAQC
Global Empire Manufacturing Corporation
職位描述
福利待遇
法定福利
13薪
健康保險
健康維護組織
QA/QC Specialist
We are seeking a highly motivated and detail-oriented QA/QC Specialist to join our dynamic team. The ideal candidate will play a crucial role in ensuring that our products meet the necessary quality standards and regulatory requirements. As a QA/QC Specialist, you will work closely with various departments to uphold our commitment to quality throughout the manufacturing process. A background in pharmaceuticals or chemistry, along with relevant licensing, is essential for this role.
Key Responsibilities
1. Quality Assurance
- Develop, implement, and maintain quality assurance policies and procedures in accordance with local and international standards.
- Conduct regular audits of production processes and documentation to ensure compliance with food safety regulations and GMP (Good Manufacturing Practices).
- Collaborate with cross-functional teams to identify areas for improvement and ensure corrective actions are implemented effectively.
2. Quality Control
- Perform routine testing and analysis of raw materials, in-process samples, and finished products to ensure quality and safety.
- Maintain accurate records of testing results, including non-conformances and corrective actions taken.
- Monitor and evaluate product specifications to ensure they meet regulatory requirements and customer expectations.
3. Regulatory Compliance
- Stay up-to-date with relevant regulations (FDA, HALAL, ISO, etc.) and ensure the company is compliant with all quality-related laws and standards.
- Prepare and submit necessary documentation for regulatory approvals and certifications.
4. Training and Development
- Provide training and support to staff on quality control processes and practices.
- Foster a culture of quality awareness and continuous improvement within the organization.
5. Reporting
- Prepare detailed reports on quality metrics, trends, and surveillance findings for management review.
- Actively participate in root cause analysis and risk management discussions.
Qualifications
- Bachelor’s degree in Chemistry, Pharmacy, or related field; must be licensed.
- Minimum of 2 years of experience in quality assurance and quality control, preferably in food or pharmaceutical manufacturing.
- Strong understanding of food safety standards and regulatory requirements.
- Excellent analytical and problem-solving skills with keen attention to detail.
- Proficient in using quality management software and tools.
- Strong communication skills, both written and verbal, with the ability to convey complex information clearly.
- Ability to work collaboratively in a fast-paced start-up environment.
- Available to start ASAP.
Corton Christian
HR ManagerGlobal Empire Manufacturing Corporation
今天回覆 1 次
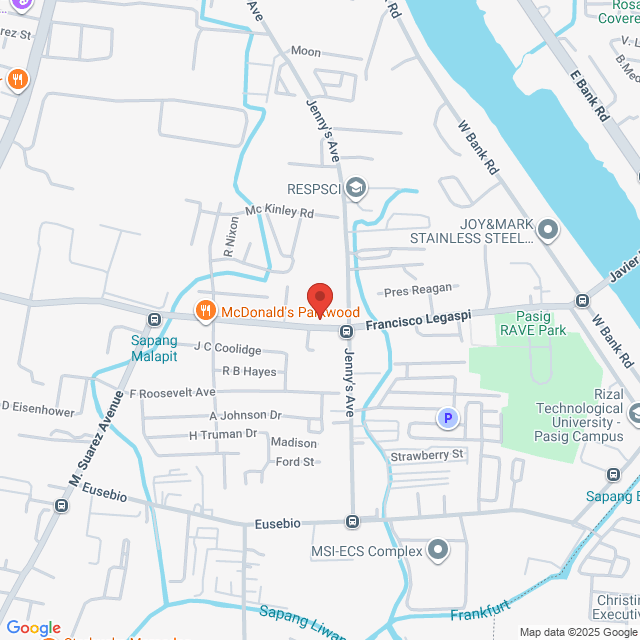
工作地址
GEM Corp. 8 Francisco Legaspi, Pasig, 1607 Metro Manila, Philippines
發布於 06 December 2025
猜你想看
查看更多Quality Assurance Tester
 Panoptik Global
Panoptik GlobalHK$6.6-10.5K[月薪]
现场办公 - 馬卡蒂5 - 10 年經驗本科全職

Recruiter HRHR Officer
Quality Assurance
 Contracting Outsourcing Management Services Incorporated
Contracting Outsourcing Management Services IncorporatedHK$2.6-2.9K[月薪]
现场办公 - 奎松市1-3 年經驗本科全職

Agunos RenalynHR Officer
QA Tester
 Tradex Network Inc.
Tradex Network Inc.HK$3.3-4K[月薪]
现场办公 - 帕賽1-3 年經驗本科全職

Network Trade XHR Manager
QA Tester
 Columbia International Food Products
Columbia International Food ProductsHK$2.6-4K[月薪]
现场办公 - 納沃塔斯1-3 年經驗本科全職
Filoteo RuthHR Officer
Structured Cabling QA & Tester
 Cablekon Engineering Services
Cablekon Engineering ServicesHK$2.6-3.3K[月薪]
现场办公 - 奎松市3 - 5 年經驗本科全職

Services Cablekon EngineeringOwner

