Regulatory Assistant
Keiko Food Corp.
職位描述
福利待遇
法定福利
13薪, 員工貸款, Pag-Ibig 基金, 菲爾健康, SSS/GSIS
健康保險
健康維護組織
其他
企業社交活動
- Prepare and submit LTO applications (initial, renewal, or variation) through the FDA ePortal
- Compile and submit complete CPR dossiers in compliance with FDA AO 2014-0029 and relevant regulations
- Coordinate with third-party laboratories for product testing (e.g., microbiological, physico-chemical)
- Monitor application status via FDA eServices and respond to FDA queries or deficiencies
- Maintain an updated compliance tracker for LTO and CPR expirations, renewals, and ongoing applications.
- Ensure that product labels, inserts, and promotional materials comply with FDA regulations
- Liaise with regulatory bodies such as BAI (for animal products), DA, or DOH as needed
- Assist in FDA inspections, audits, and document control for quality management
- Bachelor’s degree in Food Technology or related field
- At least 1 yr of hands-on experience in FDA product registration and LTO process
- Strong knowledge of FDA Circulars, AO and e-portal submission system
- Familiarity with product classification, risk categories and labeling standards
- Excellent documentation, communication, and organizational skills
- certification in HACCP, GMP, Halal is an advantage
Silang Regina
HR ManagerKeiko Food Corp.
活躍於三天內
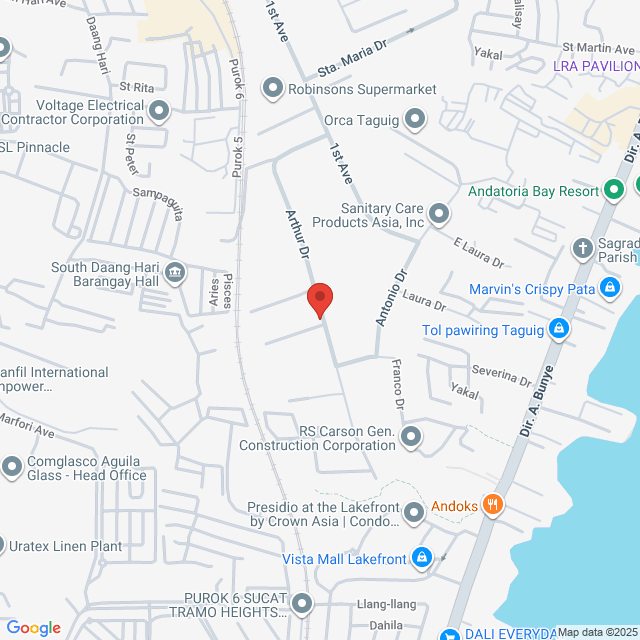
工作地址
F3C3+44R, 37 Arturo Drive, Sta. Maria Industrial Estate, Taguig, 1632 Metro Manila, Philippines
發布於 08 August 2025
猜你想看
查看更多Regulatory Officer - Regulatory Pharmacist - Fresh Grad - Greenhills, San Jua...
 Dempsey Resource Management Inc.
Dempsey Resource Management Inc.HK$2.7-3.4K[月薪]
现场办公 - 聖胡安應屆畢業生/學生本科全職
Refugia Aisa
Regulatory Officer急招
 Digital Nuclear Diagnostics, Inc.
Digital Nuclear Diagnostics, Inc.HK$2.7-3.4K[月薪]
现场办公 - 聖胡安1-3 年經驗本科全職

Samson Paul AnthonyAdmin Manager
Drug Regulatory Affairs Project Based
 LSERV Corporation
LSERV CorporationHK$3.4-4.1K[月薪]
现场办公 - 馬卡蒂<1 年經驗本科全職

Sta. Rita JhummieRecruiter
Regulatory Affairs Specialist
 Exxel Prime Int'l Trading Inc.
Exxel Prime Int'l Trading Inc.HK$2-3.4K[月薪]
现场办公 - 卡洛奧坎應屆畢業生/學生本科全職

Dagdag EsteveHR Assistant Supervisor
Regulatory Affair Specialist
 SaladStop!
SaladStop!HK$3.4-4.8K[月薪]
现场办公 - 馬卡蒂1-3 年經驗本科全職

Bernard MeceHR Assistant

