Regulatory Affairs Pharmacist Manager - 5 yrs Experience
Dempsey Resource Management Inc.
職位描述
福利待遇
員工表彰與獎勵
績效獎金
法定福利
13薪, 員工貸款, Pag-Ibig 基金, 帶薪假, 菲爾健康, SSS/GSIS
休假和請假
育兒假, 病假, 休假
Compliance and Control:
- Register our cosmetics, drugs, food supplements, medical device and household hazardous product at FDA.
- Prepare for initial or renewal of LTO licenses and coordinate with the FDA inspector
- Handle and resolve Notice of Compliance from FDA
- Maintain current knowledge of relevant regulations in Cosmetics and keeping up to date with the changes in regulatory legislation and guidelines.
- Ensures that formulation confirms to Philippine FDA standards and is within the approved limit.
- Ensures that assigned warehouses are compliant with FDA requirements.
- Conducts product test for all finished goods distributed and imported by the company.
- Schedules and facilitate monthly warehouse visit and coordinate the schedule of pest control
Customer Relations:
- Assist in updating related departments and the management on latest regulatory requirements and FDA advisories.
- Conducts product test and investigate product complaints.
- Assists in answering customer inquiries and concerns.
Suppliers:
- Assigned to handle products from imported and local suppliers.
- Conducts annual suppliers and toll manufacturer audits.
- Coordinates with manufacturers for Product Information File for filing based on ASEAN Cosmetics Directive.
- Process initial and renewal of memorandum of agreements from manufacturers.
- Request for updated GMP and ISO from manufacturers.
Requirements:
- Bachelor's degree in Pharmacy or related field.
- Experience in regulatory affairs within the pharmaceutical industry.
- Strong knowledge of FDA, EMA, and other regulatory bodies.
- Excellent written and verbal communication skills.
- Attention to detail and strong organizational skills.
- Ability to work independently and as part of a team.
- Proficiency in regulatory submission software and databases.
- Understanding of clinical trial regulations and processes.
1. Expected salary: Regulatory Manager : 40-45K
2. Work onsite : Caloocan
3. Work Monday to Friday 8:30am - 5:30pm
4. Need to know if He or She has experience in LTO inspection and release of CPR
5. Availability to Report
Refugia Aisa
Dempsey Resource Management Inc.
今天回覆超過十次
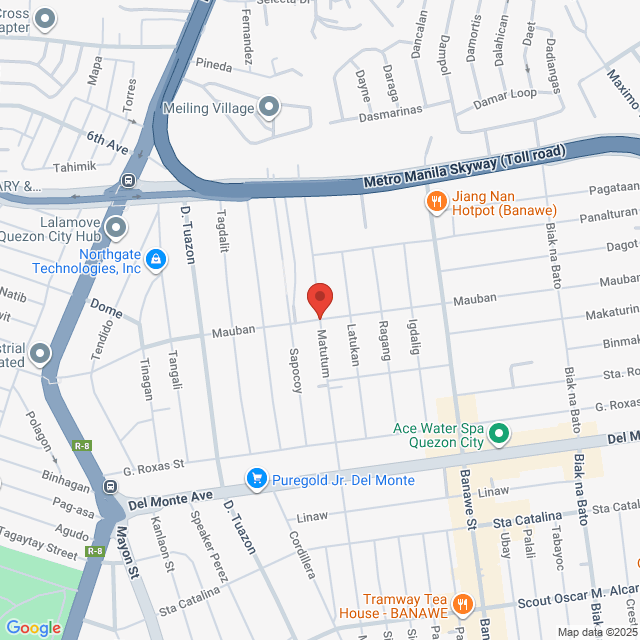
工作地址
Barangay San Jose , Quezon City. 71 Mauban, Quezon City, Kalakhang Maynila, Philippines
發布於 20 October 2025
猜你想看
查看更多Regulatory Pharmacist
 Main Source Manufacturing and Trading Corp.
Main Source Manufacturing and Trading Corp.HK$2.6-3.3K[月薪]
现场办公 - 卡洛奧坎1-3 年經驗本科全職

AND TRADING CORP MAIN SOURCE MANUFACTURINGHR Manager
Regulatory Pharmacist
 Lifestrong Marketing Inc.
Lifestrong Marketing Inc.HK$3.3-4K[月薪]
现场办公 - 卡洛奧坎<1 年經驗本科全職

Cauilan Angel JoyTalent Acquisition Associate
Regulatory Affairs Pharmacist Associate - 2 years Experience
 Dempsey Resource Management Inc.
Dempsey Resource Management Inc.HK$3.3-3.7K[月薪]
现场办公 - 卡洛奧坎1-3 年經驗本科全職
Refugia Aisa
Regulatory Affairs Pharmacist
 Allegens Healthcare Philippines Inc.
Allegens Healthcare Philippines Inc.面議
现场办公 - 馬卡蒂1-3 年經驗本科全職

Tuyet Nu NguyenHR Manager
Regulatory Pharmacist
 Ambica International Corporation
Ambica International CorporationHK$2.6-4K[月薪]
现场办公 - 帕賽無需經驗本科全職
Selda RaceRecruitment Supervisor
