Regulatory Pharmacist
Lifestrong Marketing Inc.
職位描述
福利待遇
法定福利
13薪, Pag-Ibig 基金, 菲爾健康, SSS/GSIS
其他
企業社交活動
額外福利
員工折扣
休假和請假
喪假, 生日假, 產假和陪產假, 病假, 休假
- Prepares and check submission of License to Operate renewal and other inter-related regulatory correspondence with FDA.
- Timely monitoring of product, license, permit and other licenses for renewal and ensure that all FDA license, certificates, and permits are updated and valid.
- Reviews Standard Operating Procedure based on the actual activity.
- Ensures that all pertinent documents for Cosmetic, Food, Drug and Medical Device are available for FDA inspection.
- Compiles and maintain complete technical data of each product by timely update of LTO’s master file.
- Ensures reliable IPO/Trademark monitoring
- Assists in addressing all customer inquiries by providing retention and product test results within 2 working days upon request.
- To ensure that product samples of new batches are distributed for testing 7 working days upon receipt.
- To provide product testing to Purchasing Dept. 20 working days after product test distribution or before product release to the market (for urgent testing).
- Ensures that formulation conforms to FDA standards and is within the approved limit.
- Ensures that labels of new and existing items conform to FDA Standards.
- Checking of e-Portal for newly approved CPN/CPR’s. Download, file and update the Notifications Summary.
- Generates barcode and monitoring of registration on-line.
- Ensures that Vidamed warehouses are complying with FDA requirements.
- Assists FDA inspector with the needs during inspection and makes sure that company is compliant.
- Conducts product tests for all finished goods distributed and imported by the company.
- Maintain current knowledge of relevant regulations in Cosmetic/Food/Medical device/Drugs and keeping up to date with changes in regulatory legislation and guidelines.
- Assists in updating related departments and regulatory head on latest regulatory requirements and FDA advisories.
- Coordinates the schedule of pest control.
- Monitoring in conducting annual audits of all active suppliers.
- Coordination with manufacturers for Product Information File for filing based on ASEAN Cosmetic Directive.
- Conducts a Product Information File for filing based on ASEAN Cosmetic Directive.
- Processing of initial and renewal of agreements and request for updated GMP and ISO of all active suppliers.
- Perform other related duties as required by FDA and other tasks assigned as needed.
Cauilan Angel Joy
Talent Acquisition Associate Lifestrong Marketing Inc.
活躍於三天內
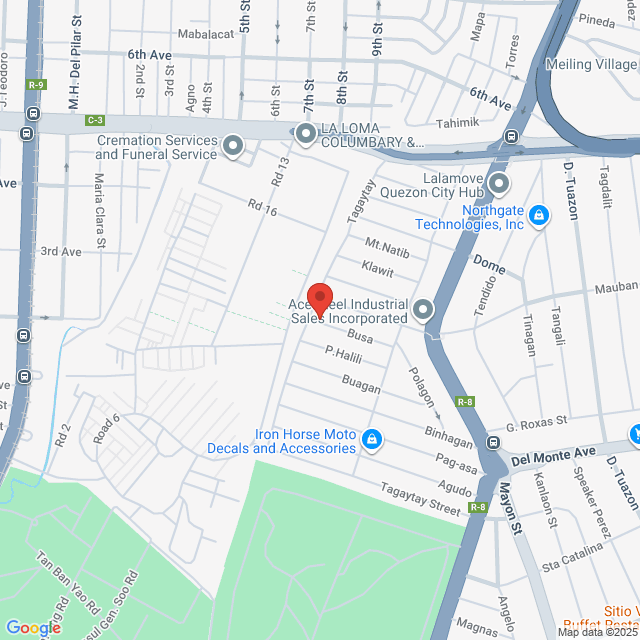
工作地址
98 Busa, Caloocan, 1404 Metro Manila, Philippines
發布於 22 October 2025
猜你想看
查看更多Regulatory Pharmacist
 Main Source Manufacturing and Trading Corp.
Main Source Manufacturing and Trading Corp.HK$2.6-3.3K[月薪]
现场办公 - 卡洛奧坎1-3 年經驗本科全職

AND TRADING CORP MAIN SOURCE MANUFACTURINGHR Manager
Regulatory Affairs Pharmacist Manager - 5 yrs Experience
 Dempsey Resource Management Inc.
Dempsey Resource Management Inc.HK$5.3-6K[月薪]
现场办公 - 卡洛奧坎5 - 10 年經驗本科全職
Refugia Aisa
Regulatory Affairs Pharmacist
 Allegens Healthcare Philippines Inc.
Allegens Healthcare Philippines Inc.面議
现场办公 - 馬卡蒂1-3 年經驗本科全職

Tuyet Nu NguyenHR Manager
Regulatory Pharmacist
 Ambica International Corporation
Ambica International CorporationHK$2.6-4K[月薪]
现场办公 - 帕賽無需經驗本科全職
Selda RaceRecruitment Supervisor
Regulatory Pharmacist
 Raya Regenerative and Preventive Medicine
Raya Regenerative and Preventive MedicineHK$4-5.3K[月薪]
现场办公 - 馬卡蒂<1 年經驗本科全職

Gamboa JustineHR Associate

