Regulatory Pharmacist
Wellpro Biomedical Inc
職位描述
福利待遇
員工表彰與獎勵
績效獎金
法定福利
13薪, Pag-Ibig 基金, 帶薪假, 菲爾健康, SSS/GSIS
健康保險
健康維護組織
Your responsibilities will include:
- Prepares & submits documents to Philippine Food and Drug Administration (FDA) such as License to Operate (LTO) & drug product - ACTD document for Initial registration, Site Registration/GMP Clearance applications, BA/BE study report, renewal of CPR, including variations and compliance of existing products.
- Must know or have an experience applying Initial and renewal application for drug products.
- Monitors the status of applications with the FDA.
- Creates and reviews packaging layouts in line with existing standards set by FDA.
- Handles company inspection and audit relevant to third party and government agencies.
- Handles applications with Intellectual Property Office (IPO) for trademarks and other functions of the agency.
- Directly reports to Regulatory Manager.
To be successful in this role, you will need to have:
- Bachelor’s Degree in Pharmacy or any related courses in Medical Field.
- At least 2 years of working experience as Regulatory Compliance Officer.
- Detail oriented and attention to accuracy.
- Excellent communication and interpersonal skills.
Team HR
HR OfficerWellpro Biomedical Inc
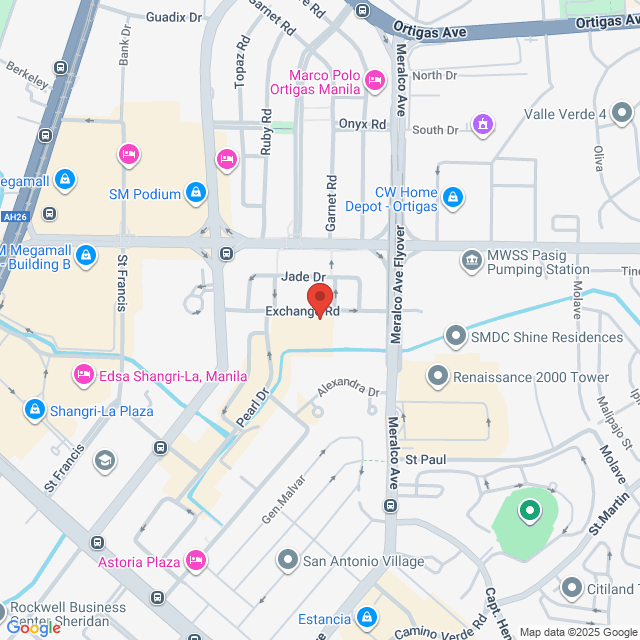
工作地址
Unit 2802-D, 28th floor, Tektite Tower - East Tower. Exchange Rd, Ortigas Center, Pasig, 1605 Metro Manila, Philippines
發布於 18 September 2025
猜你想看
查看更多Regulatory Pharmacist
 East Lane Corporation
East Lane Corporation面議
现场办公 - 巴石1-3 年經驗本科全職

hr RUCEL BAUTISTARecruiter
Regulatory Pharmacist
 Elixir Pharma Inc.
Elixir Pharma Inc.HK$2.6-4K[月薪]
现场办公 - 巴石3 - 5 年經驗本科全職

Almayda JuvelynHR Officer
Regulatory Pharmacist
 Rivermed, Inc
Rivermed, IncHK$3.3-4K[月薪]
现场办公 - 巴石1-3 年經驗本科全職

Castalone JessaHR Officer
Regulatory Affairs Pharmacist
 Allegens Healthcare Philippines Inc.
Allegens Healthcare Philippines Inc.面議
现场办公 - 馬卡蒂1-3 年經驗本科全職

Tuyet Nu NguyenHR Manager
Regulatory Pharmacist
 Ambica International Corporation
Ambica International CorporationHK$2.6-4K[月薪]
现场办公 - 帕賽無需經驗本科全職
Selda RaceRecruitment Supervisor

